HK inno.N confirmed nonclinical efficacy of its novel EGFR-mutated NSCLC treatment
April 11, 2024
HK inno.N confirmed nonclinical efficacy of its novel EGFR-mutated NSCLC treatment
Nonclinical efficacy findings of the next-generation allosteric EGFR-TKI candidate (study name: IN-119873) presented at the AACR Annual Meeting in the U.S.
IN-119873 demonstrates excellent efficacy in models of EGFR mutation resistant to major drugs and brain metastasis
Rising expectation for synergistic effect with 3rd-generation EGFR-TKI as a combination therapy
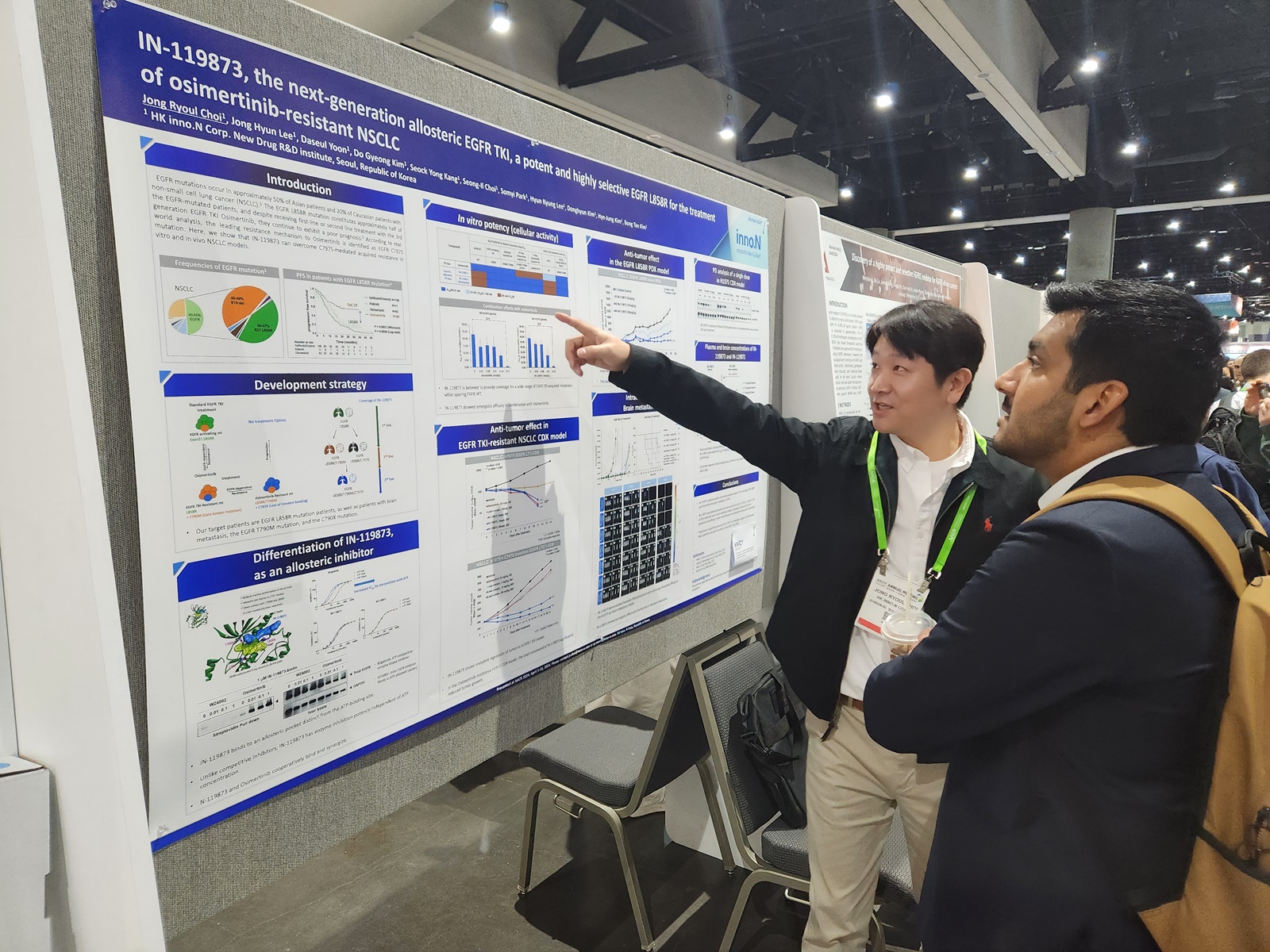
HK inno.N announced on April 11 that its poster presentation introducing the nonclinical research findings on its next-generation allosteric Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor (EGFR-TKI) candidate was well received at the ‘American Association for Cancer Research (AACR) 2024’ Annual Meeting on April 8 (local time).
According to the efficacy outcomes published by HK inno.N, 'IN-119873' exhibited remarkable efficacy against major drug-resistant EGFR resistance mutations (EGFRT790M double mutations and EGFRC797S triple mutations), including the L858R mutation, and demonstrated notable effectiveness in a brain metastasis model.
Furthermore, when combined with osimertinib, an existing third-generation EGFR-TKI, ‘IN-119873’ demonstrated stronger binding to EGFR mutations. Its minimal inhibitory activity on normal EGFR allows reduced side effects associated with existing EGFR-TKIs.
Approximately half of the patients with EGFR-mutated non-small cell lung cancer (NSCLC) present EGFR L858R mutation, which has been reported to have a poor prognosis despite treatment with Osimertinib HK inno.N’s ‘IN-119873’ is expected to reduce side effects and improve efficacy through combination therapy with Osimertinib.
“Our planned timeline for development is to complete the ongoing nonclinical research on ‘IN-119873’ and apply for the Investigational New Drug (IND) for entry into clinical stage (phase 1) within this year,” said Kim Bong-tae, the director of the HK inno.N Novel Drug Research Center, adding, “‘IN-119873’ is expected to provide a new therapeutic option for patients with NSCLC refractory to standard treatments.”
HK inno.N is continuing to research on ‘IN-119873’, a 4th-generation targeted anticancer drug candidate for NSCLC patients with the EGFR L858R mutation who are resistant to conventional first-line treatments. HK Innoen's 'IN-119873' targets the allosteric binding site (one of the protein binding sites) of EGFR, unlike existing treatments that target the adenosine triphosphate (ATP) binding site of EGFR. With this novel approach to treatment for NSCLC, ‘IN-119873’ is highly expected to demonstrate excellent efficacy against the EGFR mutations caused by existing 1st-, 2nd- and 3rd-generation EGFR-TKI treatments and synergize with 3rd-generation EGFR-TKI as a concomitant therapy. [The End]
[Reference information]
Title of the poster presentation: IN-119873, the next-generation allosteric EGFR TKI, a potent and highly selective EGFR L858R for the treatment of osimertinib-resistant NSCLC.
▶EGFR: EGFR stands for Epidermal Growth Factor Receptor. When binding to its receptor, the EGF is involved in cell growth and differentiation through signaling. Any abnormality in the EGFR’s function, such as overexpression, is known to affect the development and metastasis of various tumors. Therefore, anticancer drugs are being developed using a mechanism by which EGFR activities are inhibited.
▶TKI: TKI stands for Tyrosine Kinase Inhibitor. Tyrosine kinases are a family of enzymes responsible for cell signaling, growth and division, etc. In some cancer cells, these enzymes are overactive or present at a high concentration; therefore, interfering with such activities is helpful in inhibiting the growth of cancer cells. Accordingly, TKIs are used to treat cancers as a type of targeted therapeutics.
▶ATP: ATP stands for Adenosine Triphosphate, which means an organic compound made up of three phosphate groups attached to an adenosine base. A paradigm for conventional EGFR-TKIs is to block the binding to ATP, the energy source for cancer cells.
▶Osimertinib (ingredient name): First-line treatment for locally advanced/metastatic NSCLC patients with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation.