Only ethical management based on CP No. 1 management philosophy is the path to hope for becoming a 100-year-old company.
In addition, we will expand close third-party management and compliance operation dissemination to grow together with stakeholders.
I promise you that we will continue to make efforts to deliver greater trust and value.
Self-Compliance Manager
CEO
Kwak Dal-won

We will spread the compliance culture and grow into a CP model company that is continuously recognized both internally and externally.
Autonomous compliance manager
Strategy Support Office Director
Kim Ki-ho

Our commitment to fair trade practices
-
We abide by the law and internal guidelines for fair competition in the market for pharmaceutical products.
-
We do not offer illegal compensation in exchange for the purchase of our products.
-
We prioritize the interests of patients and make concerted efforts to improve public health.
-
We aim to become a global pharmaceuticals firm that grows in a sustainable manner.
What is the compliance program for fair trade?
Compliance Program
Code of conduct and vision
Only through ethical management can we achieve a genuinely affluent future.
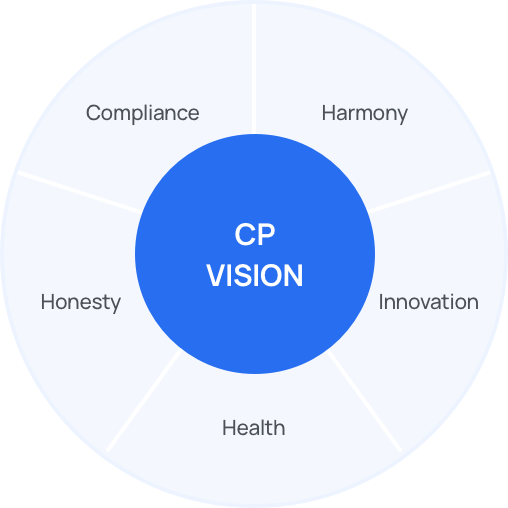
-
Health
We prioritize the interests of patients and make concerted efforts to improve public health. -
Honesty
We do not engage in any illegal acts, and in doing so work towards a society based on honesty and integrity. -
Compliance
We abide by relevant laws and internal guidelines to help establish fair competition in the market for pharmaceutical products. -
Harmony
We are an ethical company that lives up to its social responsibility and exists in harmony with the local community. -
Innovation
We aim to become a truly global player with our compliance program.
System for compliance implementation
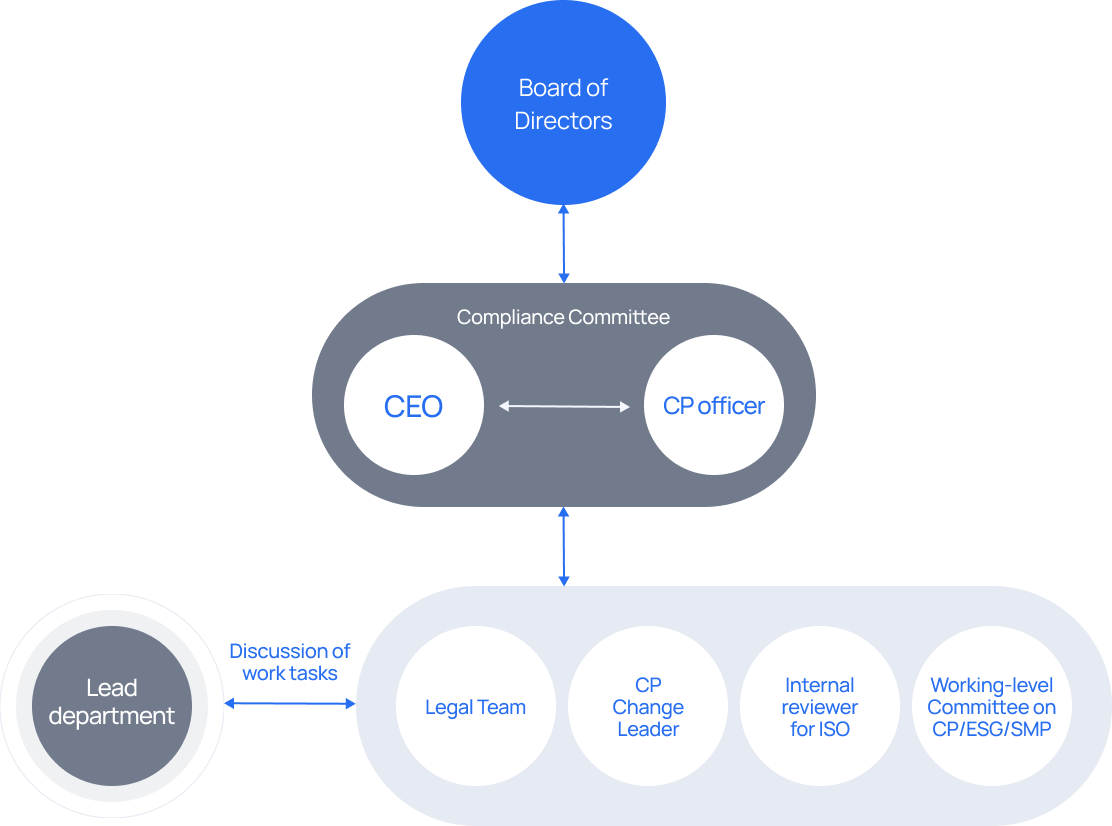
-
Compliance Committee
Decision-making body on compliance implementation
- Chair: CEO
- Members of the Committee:
CP officer, senior executives, heads of relevant departments -
Working-level Committee on CP/ESG/SMP
Working-level Committee, communication channel
Providing CP guidelines and the result of monitoring -
Compliance team
CP opreration, postulation to HR Committee regarding violations -
ESG management team
Operation of CP Change Agent & Leader
Management of internal reviewer for ISO -
HR Committee
Sanctions for issues of non-compliance (HR Team, etc.)
History of HK inno.N's Compliance Program
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015
- 2014
- Before
establishment of
HK inno.N
-
JanuaryConducted CP operation survey targeting ETC Sales & Marketing Headquarters
Revised CP guidelines and detailed operation manual (1/2)
Published the 2025 first-half Fair Trade Compliance Handbook (1/6)
CEO instructed the creation of the "CP Fair Trade Song"FebruaryDisclosed the status of the Fair Trade Compliance Program operation (2/11)
Conducted stakeholder importance assessment (employees, customers, investors, media, government, local communities, etc.)
Internal training on Fair Trade Act and internal transactions (2/14)
External training: Key amendments to the Fair Trade Act and implications for business planning [Korea Fair Competition Federation] (2/20)
External training: 2025 Fair Trade Policy Direction [Fair Trade Commission Competition Policy Division] (2/27)MarchHeld the 2025 Compliance Day ceremony
Awarded 2024 CP compliance excellence prizes
Conducted CP training for compliance managers at nationwide sales offices (March–April)
Launched first-half expense report self-check campaignAprilPosted the 2025 first-half CEO CP compliance message posters at nationwide offices (4/7)
Attended Compliance Manager Roundtable [Korea Fair Competition Federation] (4/9)
Broadcasted the "CP Fair Trade Song" at all business sites (4/7)
Conducted advanced training on CP compliance risk assessment (4/28–29)MayInvited external expert for training: Corporate Secret Protection and Practices - CEO and all employees (5/22)
Fulfilled mandatory CSO reporting system training for new promotional sales staff (5/29)
Conducted CP compliance risk assessment (1st round) - Identified risks related to Fair Trade laws through department interviews
Attended KPBMA 80th Anniversary Ethical Management Workshop (5/30)JuneConducted 2025 survey - Submitted 2024 expense report details (6/20)
Conducted CP training for third parties (June–July)
External training: 2025 Fair Trade Trends and Case Seminar [Law Firm Gwangjang] (6/17)JulyReported the effectiveness evaluation of CP operations for the first half of 2025
Published second-half organizational compliance handbooks
Launched second-half expense report self-check campaign
Conducted Fair Trade Commission-related law training for executives - CEO and senior management (7/24)AugustPosted the 2025 second-half CEO CP compliance message posters at nationwide offices
Conducted CP training for third parties
Conducted CP compliance risk assessment (2nd round) - Verified departmental control measures and improvement plans for Fair Trade law risks (August–September)
Improved the internal whistleblowing system "Ethical Management Reporting"SeptemberReported first-half CP operation effectiveness to the board of directors (9/1)
Published Fair Trade Commission-related law case CP monthly magazine: "CP Zoom-In" (first issue)
Conducted the 19th full revision of the Fair Trade Compliance Program operation regulations and guidelines (9/24)
Invited external expert for training: U.S. Compliance Risk - CEO and senior management (9/25)
Established new format for promotional sales staff training certificates (9/26)
Attended the 2025 APEC Ethical Business Forum
Held the 2025 CP Creative Shorts ContestOctoberPublished new compliance handbook for suppliers
Developed and distributed five types of "Fair Trade Compliance Checklists" (10/31)
Reported results of CP operation system enhancement (10/31)NovemberConducted cross-training for CP Change Leader & Agent 4th cohort (November–December): FCPA
Conducted company-wide CP TEST
Conducted CP compliance risk assessment (3rd round) - Reviewed risk evaluation guideDecemberEstablished new procedures for verifying expense reports of retirees (12/1)
Launched new sales system (N.sales) (12/1)
Reported Fair Trade Commission-related law operation system results and 2026 annual plan - CEO and senior management (12/5)
Awarded 2025 CP compliance excellence prizes (12/5)
Expanded and reorganized CP operation regulation guidelines (12/18)
Established and enacted guidelines for the autonomous dispute resolution organization (12/18)
Distributed CP letters to third parties
Conducted CP compliance risk assessment (4th round) - Provided legal education for high-risk departments
Produced CP office supplies (clear files) and distributed them to nationwide offices (CP Code of Conduct & Fair Trade Act DO & DON'T) -
JanuaryConducted company-wide assessment on the effectiveness of CP operationsFebruaryOpening of social media CP channel「inno.N CP Talk」
Conducted stakeholders materiality assessment(executives and employees, customers, investors, media/press, government, community, etc.)MarchPledge ceremony for Anti-Corruption and Compliance(To commemorate the Day of Compliance, all executives and employees proceed at Osong headquaters)AprilHeld the 100th CP Committee(award ceremony for outstanding CP employees)
2024 Company-wide CP poster campaign led by the CEO
Signed a Letter of Pledge for Anti-Corruption and Compliance(all executives and employees)
Distributed CP Handbook
CP training for Sales Unit nationwide(April to May)
Round-table meeting with CP Officer for each division(April ~ November)MayAttend to Corporate Integrity Forum(UNGC-NBIM)JuneIssued 2024 CP Manual
Revised CP Regulations and Operational Guidelines
JulyRevised CP Guidelines and Revision of CP Guidelines and Operations Manual
Submitted an inspection report for implementation of 2023 Expenditure ReportsAugustTraining on 'Importance of supply chain risk management' with invited speakers of experts for CP Change Leaders & AgentsOctoberConducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N : Human rights management
Distributed CP Letter for Third PartiesNovemberLaunched the Expenditure Reports Self-audit Campaign
Hosted a company-wide CP TEST
Special CP training(with invited speakers)DecemberETC team for Award ceremony for outstanding CP
Held CP Training Program for Third Parties
Achieved the rating "AA" in the corporate CP assessment (the Korea Fair Trade Commission) -
JanuaryConducted company-wide assessment on the effectiveness of CP operations
Revised CP Regulations and Operational GuidelinesFebruaryIssued 2023 CP Manual
Round-table meeting with CP Officer for each division (February to November)
Conducted stakeholders materiality assessment (executives and employees, customers, investors, media/press, government, community, etc.)MarchTraining on ESG Management with invited speakers of CP experts for CP Change Leaders & Agents
Held a ceremony for the Day of Compliance (CP Contest and awarding winners, award ceremony for outstanding CP employees)April2023 Company-wide CP poster campaign led by the CEO
Revised CP Regulations and Operational Guidelines
Revised CP Guidelines and Operations Manual
Signed a Letter of Pledge for Anti-Corruption and Compliance (all executives and employees)
Conducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N (the 1st half of 2023): ESG Management (Environment)MayEstablished an online application system for Expenditure ReportsJuneSubmitted an inspection report for implementation of Expenditure Reports
Revised CP Guidelines and Revision of CP Guidelines and Operations Manual
Held CP Training Program for Third PartiesAugustDistributed CP Letter for Third PartiesSeptemberLaunched the Expenditure Reports Self-audit CampaignOctoberTraining on Compliance and Corporate Investment with invited speakers of CP experts for CP Change Leaders & AgentsNovemberEstablished the monitoring SOP
Special CP training (with invited speakers)
Conducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N (the 2nd half of 2023): ESG Management (Social), socially responsible management
Hosted a company-wide CP TESTDecemberRevised CP Regulations and Operational Guidelines
All members who completed the CP Change Agent course (3rd) were accepted to CP Change Leader course -
JanuaryConducted company-wide assessment on the effectiveness of CP operationsFebruaryTraining on FCPA with invited speakers of CP experts for CP Change Leaders & Agents
Conducted stakeholders materiality assessment (executives and employees, customers, investors, media/press, government, community, etc.)MarchHeld a ceremony for the Day of Compliance (CP Contest and awarding winners, award ceremony for outstanding CP employees)
Enacted Compliance Policy (March 10, board approval)April2022 Company-wide CP poster campaign led by the CEO
Distributed CP Handbook
Held CP Training Program for Third Parties
Signed a Letter of Pledge for Anti-Corruption and Compliance (all executives and employees)
Conducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N (the 1st half of 2022): FCPA
Training on ESG with invited speakers of CP experts for CP Change Leaders & AgentsMayConducted CP training for Third PartiesJuneRound-table meeting with CP Officer
Submitted UN Global Compact (UNGC) Progress Report (Communication on Progress: COP)JulyConducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N (the 2nd half of 2022): General principles of ESG managementOctoberThe 14th Revision of the HK inno.N CP RegulationsNovemberRound-table meeting with CP Officer
Hosted a company-wide CP TESTDecemberCP training for Sales Unit nationwide on Expenditure Reports
Distributed CP Letter for Third Parties -
JanuaryConducted a company-wide survey on CP awareness
Creation and distribution of the CP Pocket Guide BookFebruaryFCPA (Foreign Corrupt Practices Act) training with invited speakers of CP experts for CP Change Leaders & Agents
Held a company- wide FCPA training with invited speakers of CP experts (a total of 618 attendees combing online and offline channels)MarchHeld a ceremony for the Day of Compliance
Held the CP Contest (Design figures/characters, slogans) and awarded the winners
Held an annual award ceremony for outstanding CP employees (17 employees)AprilRevised CP Guidelines and Operations Manual
The 12th Revision of the HK inno.N CP RegulationsMayConducted a cross-training among CP Change Leaders & Agents for all executives and employees of HK inno.N: FCPAJuneTraining on collusion with invited speakers of CP experts for CP Change Leaders & Agents
Revised CP Guidelines and Operations ManualAugustHeld special CP training - for ETC Sales & Marketing managersSeptemberConducted training by CP Change Leaders & Agents for all executives and employees of HK inno.N: CollusionOctoberHeld special CP training - for production managers
Conducted training with invited speakers of CP experts for CP Change Leaders & Agents : ISO 37301NovemberHosted a company-wide CP TEST
Conducted a cross-training by CP Change Leaders & Agents for all executives and employees of HK inno.N: ISO 37301
Performed training effectiveness assessment on the CP Change Leader&Agent programDecemberAchieved the rating "AA" in the corporate CP assessment (the Korea Fair Trade Commission)
All members who completed the CP Change Agent course were accepted to CP Change Leader course
Conducted a CP Change Leader Promotion Test -
FebruaryRenewal of CP Letter (Improved accessibility/readability for the letter including application of QR code)MarchAwarded outstanding CP employees and outstanding CP branch offices (17 employees/16 teams)
CP online training for ETC sales headquartersMayHeld a CP Video Contest and awarded the winnersJuneRound-table meeting with CP Officer
CP training for Third Parties (7 companies including wholesalers)JulyThe 2nd training course for CP Change Leader & AgentAugustCirculated a company-wide CP No.1 templateOctoberThird-party CP trainingNovemberThird-party CP training
Hosted a company-wide CP TESTDecemberDistributed CP Letter for Third Parties -
FebruaryHeld the 1st CP Change Agent Training Course in 2019
CP training for Third PartiesMarchHeld the 4th ceremony and events of the Day of Compliance
Held an award ceremony for outstanding CP employeesAprilIssued the 100th CP LetterMayDisseminated CP best practices of HK inno.N for companies in the pharmaceutical industryJuneThe 2nd CP Change Agent Training Course in 2019JulyDeveloped a mobile expense processing systemOctoberThe 3rd CP Change Agent Training Course in 2019NovemberHosted a CP TEST for executive and employees of Ethical Drug (ETC) DivisionDecemberThe 4th CP Change Agent Training Course in 2019
All members who completed the CP Change Agent course were accepted to the Leader course (20 ppl in total) -
JanuaryLaunched the Expenditure Reports System
The 1st CP training for Third Parties in 2018
CP training for expatriate employeesFebruaryFormulated details for CP commendation rules
The 2nd CP training for Third Parties in 2018MarchHosted CP Spring Writing Contest and held an award ceremony for winners
The 3rd CP training for Third Parties in 2018 with the CP OfficerAprilHeld the 3rd ceremony and events of the Day of Compliance
Signed and collected a Letter of Pledge for Compliance (all executives and employees)
Presented CP best practices of HK inno.N at the CP Forum of Korea Fair Competition Federation (1st half of 2018, CP Officer)
Formed a Compliance Supervision UnitMay~JuneThe 4th CP training for Third Parties in 2018
Disseminated CP best practices of HK inno.N for companies in other industries (May) and the pharmaceutical industry (June)AugustCollected CP MOU from Third PartiesNovemberEstablished CP Change Agent Training Course as the first case in the industryDecemberDistributed/circulated 2018 CP Manual Version 4 -
JanuaryImplemented division-specific risk assessment modelsMarchHeld CP Contest for building a strong CP-oriented culture (Slogans, design figures/characters)
On-site CP training for Third Parties (Jeju)
Conducted internal inspection/audit in relation to Fair Agency Transactions Act
Distributed CP Pocket GuidelinesAprilHeld the 2nd ceremony and events of the Day of Compliance
Signed a Letter of Pledge for Compliance (all executives and employees)
2017 nationwide CP Poster campaignMayThe 2nd On-site CP training for Third Parties (Jeonju)JuneDistributed/circulated 2017 CP Manual Version 3
Board approval of the complete revision of CP RegulationsJulyEstablished a whistleblowing system within the company websiteSeptemberBuilt a task force team for development of the Expenditure Reports System and conducted intensive trainingOctoberCompany-wide training following the 4th revision of the Fair Competition CodeDecemberAchieved the rating "AA" in the corporate CP assessment
Completed the development of the Expenditure Reports System -
AprilEstablished the Day of Compliance
Implemented the Risk Assessment Model(HK inno.N Ethical Management Model)JuneEstablished the CP Working Group (Steering Committee)
Distributed/circulated 2016 CP Manual Version 2OctoberExpanded the Compliance team, Distributed guidelines on the Improper Solicitation and Graft ActNovemberThe CP Officer acquired the Certified Compliance Professional (CCP) certificateDecemberKang Seok-hee (then CEO) was jointly appointed as the CP Officer
Developed division-specific risk assessment models -
JanuaryLaunched circulation of CP LetterAprilConvened CP CommitteeAugustEstablished Standard Operating Procedures (SOPs) for the CP Management TeamSeptemberDeveloped a Risk Assessment Model (HK inno.N Ethical Management Model)OctoberDistributed 2015 CP Manual Version 1
-
AprilFounded HK inno.N Corporation (Material division to CJ Healthcare) and established HK inno.N Compliance Program (CP) RegulationsMayFormed a CP task-force team (TFT) of HK inno.NJuneAnnounced a strategic focus on CP and appointment of CP Officer & signing of a letter of pledge for CPJulyAttended Corporate Ethics Charter Declaration Ceremony held by KPBMAAugustAttended APEC Ethics Forum (Nanjing, China) / Distributed HK inno.N Manual and Guideline HandbookNovemberDeveloped a Pre-Post Monitoring System (SMP:Sales & Marketing Portal)
-
2002Intrduced CJ CheilJedang Compliance Program (CP) and established CP regulations2007Announced adoption of Compliance Program for pharmaceutical industry (hosted by KPBMA /CJ CheilJedang participated)2010Human resource allocation dedicated to CP management for the pharmaceuticals division of CJ CheilJedang2014Composed a CP task force team (TFT) and CP unit within the pharmaceuticals division of CJ CheilJedang



